GLOBAL REACH WITH AN AGILE APPROACH
Ipsen is an international group, with products registered in more than 100 countries. With 5,200 employees working to create value for patients and society around the world, we have global reach while still being an agile, mid-sized company.
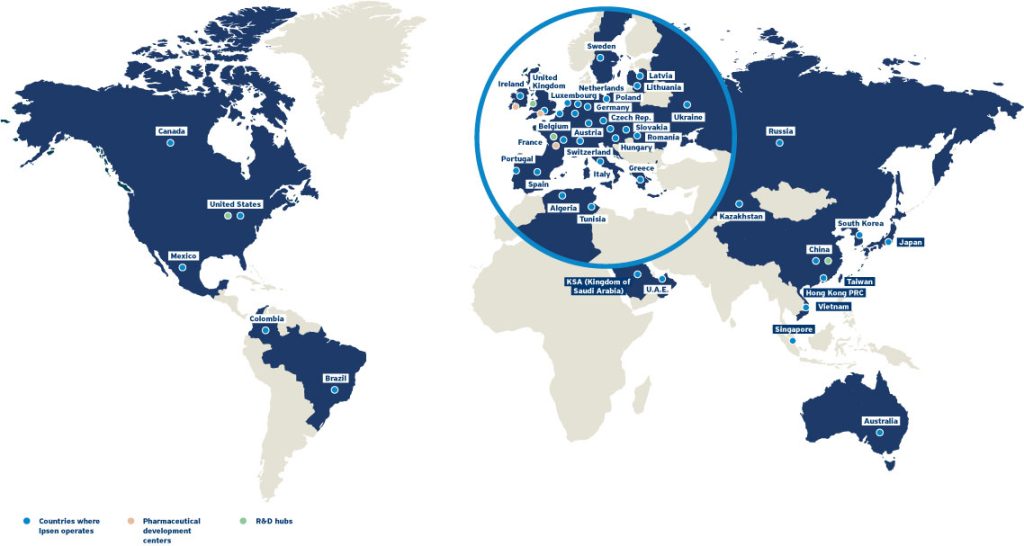
Global Presence
Our global teams are committed to tackling areas of high unmet medical need to improve the quality of life of patients and caregivers. With our science-led and patient-driven approach, we grow together in a supportive environment.
100+ countries
where Ipsen products are marketed
41 countries
where Ipsen has a direct presence
5,200+ employees
around the world
Countries where Ipsen operates
Production and R&D sites
Ipsen scientists and innovators around the world are developing life-changing treatments. Through our global network and collective expertise, we are changing patients’ lives for the better.
12M packs
produced in 2022
4 manufacturing sites
around the world
4 power bases
driving our innovation
Production and R&D sites (overview)
Our production and R&D sites around the world represent the significant investments we made in 2022 to support continuous innovation.
Paris-Saclay, Dreux and Signes
Our team at Signes specializes in the aseptic manufacturing of sustained-release injectable products. Signes also performs testing, packaging and distribution of Ipsen products, notably Somatuline® (lanreotide), Decapeptyl® (triptorelin) and Onivyde® (irinotecan liposome injection).
Wrexham and Milton Park
Following significant investment, our world-class biologic campus in Wrexham has increased its production capacity with a new building and filling line for its flexible drug product (FDPF) facility. A strategic R&D center for the development of recombinant neurotoxins, Wrexham produces and distributes Dysport® (abobotulinumtoxinA), Azzalure® (abobotulinumtoxinA) and Alluzience® (abobotulinumtoxinA) worldwide.
Cambridge and Newton, U.S., and Montreal, Canada
Placing Ipsen at the center of the biotech revolution in Cambridge, Massachusetts, our Bioscience R&D center primarily focuses on oncology and rare disease. The site, which specializes in the manufacture of Onivyde® bulk for the U.S. market, also welcomed Epizyme teams in 2022.
Beijing and Shanghai
Our Beijing site oversees clinical trial coordination in Asia while our Shanghai site develops strategies to register new indications and compounds in China.
Dublin
Our Dublin site manufactures bulk peptide active pharmaceutical ingredients (APIs) for Somatuline® and Decapeptyl®. Dublin is also a center of peptide and small-molecule product development, and the release testing for Dysport® for the EU market. In 2022, Ipsen increased treatment manufacturing in Ireland by 10% – compared to 2021 – and has an investment plan of €50 million to support continued innovation, sustainability and technology upgrades.